Serious adverse effects are real and rare
Any adverse outcome from vaccine use is a concern to everyone, from vaccine manufacturers to the people who experience them. Vaccines in a way are like cars – incredibly safe and effective, but not without some risk. We don’t worry as much about collisions in automobiles as we should, and driver inattention is a significant contributor. In both cases the risk of vaccine adverse events and collisions are to be expected and understood. One of the purposes of the work of the GVDN is to find out, when an adverse event occurs, what the reason for it is and what the risk is, compared with no vaccine. My work in GVDN is to ask this same question but find out what genetic factors contribute to adverse events.
We are all 99.9% genetically identical, but this still means there are millions of genetic differences between us.
So, what role might genetic variation play in the development of vaccine-induced adverse events?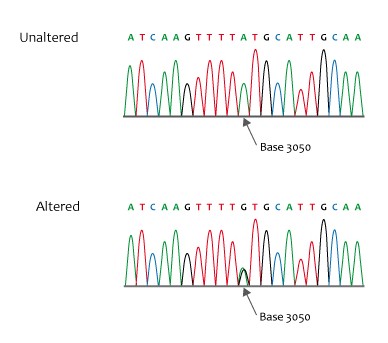
The method we will use to explore this I have been studying the genomic contribution to adverse drug reactions for nearly 20 years and started as soon as the technology was cheap enough to begin to consider genomic screening within the constraints of tight research budgets. We used case-control observational trial methods (where people who have an adverse event to a drug are compared with people who received the same drug but did not have the event). A good review of this method can he found here.
When we study vaccine safety, it is very important to make sure the cases of adverse events are real and related to the drug or vaccine. For example, if a person has chest pain and difficulty breathing, we need to differentiate between a stress event and a heart-related event such as myocarditis. Here is where the clinical notes detailing the symptoms, and laboratory and other medical test results are very important. We start with the most rigorously defined cases of adverse events and find patients who were also exposed to the drug but without the reaction. We then compare the genetic profiles between the two groups. This approach has been surprisingly successful at identifying highly associated (odds ratios ≥ 3, essentially 3x), biologically plausible genomic risk factors for a variety of serious adverse drug reactions. Some examples can be found in these papers:
Figure 1. Missense sequence
Credit: NHS National Genetics and Genomics Education Centre, CCA 2.0, via Wikimedia Commons.
- Aminkeng F, Bhavsar AP, Visscher H, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015. PMID26237429
- Kowalec K, Wright GEB, Drögemöller BI, et al. Common variation near IRF6 is associated with IFN-β-induced liver injury in multiple sclerosis. Nat Genet. 2018. PMID30013178
- Ross CJ, Katzov-Eckert H, Dubé MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009. Erratum in: Nat Genet. 2013. PMID19898482
- Visscher H, Ross CJ, Rassekh SR, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012. PMID21900104
Genetic studies need power
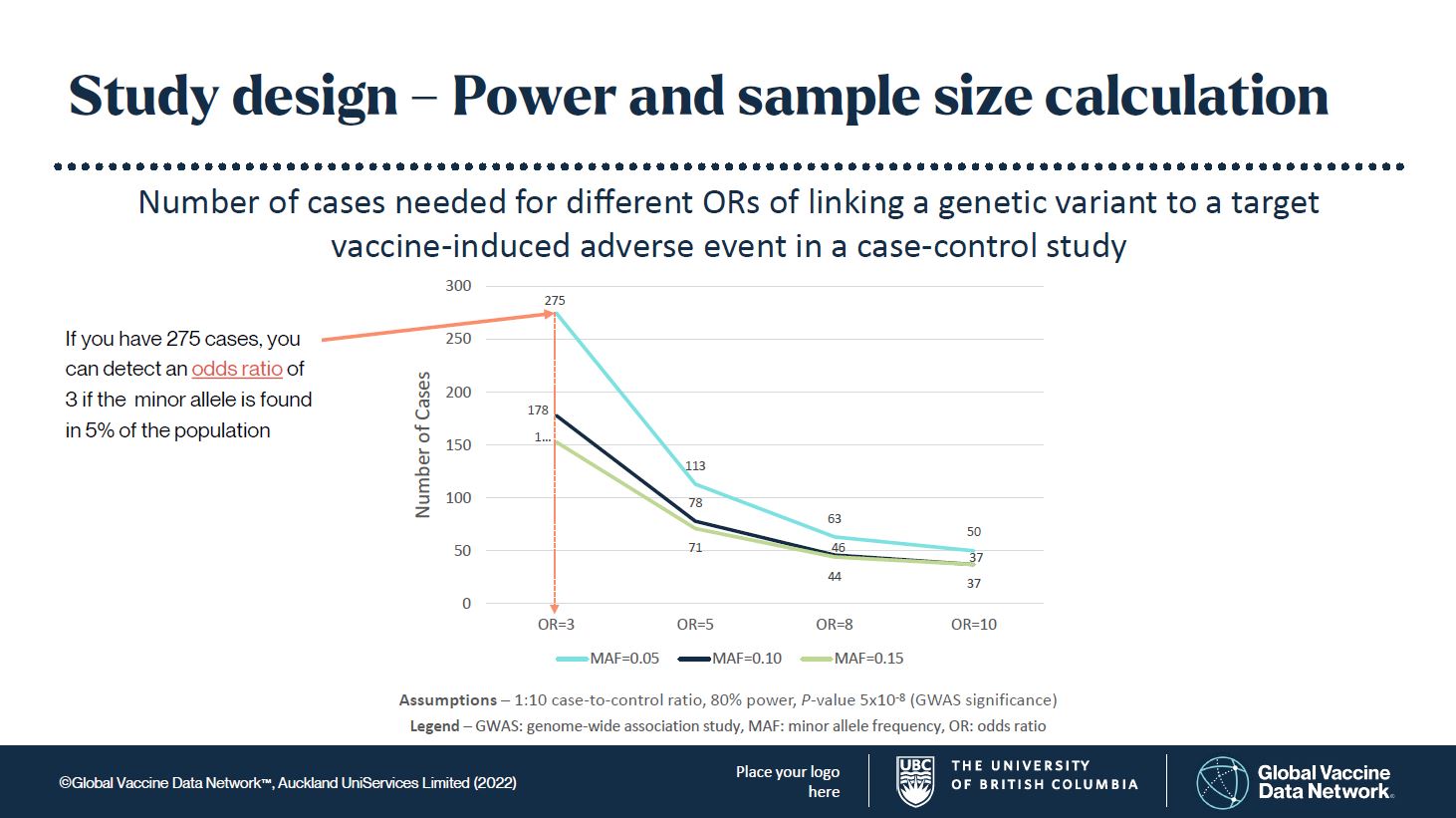
Many genetic studies are underpowered, in part because serious adverse effects are rare. Genome-wide association studies (GWAS) require statistical significance levels that reflect the large number of genetic variants to be examined. Normally, a probability value (P-value) of 0.05 is considered statistically significant, in that the result is probably correct 95% of the time. However, for these genomic studies, instead of P-values <0.05, we set an initial P-value at 5x10-8 or p = 0.00000005!
The frequency of the genetic variant, called the minor allele, is also an important consideration. If the minor allele frequency (MAF) is too low, you may never find it. Generally, I would set the expected MAF in a population at 5, 10, or 15%. This means it is common enough to find, but not rare enough that you may never find it. This has worked well in drug adverse event studies. Controls (patients without the adverse event) are matched to cases (patients with the adverse event) in large numbers to improve statistical power. From these assumptions you can see in Figure 1 that the numbers of cases needed to find something truly significant are not unreasonable to achieve.
Figure 2. (click here to download this image)
Finding the cause is the first step in finding a treatment
These biomarkers have stood up to further validation work in the drug arena by using pharmacokinetic, cell- or animal-based functional models. One specific approach we have used was recently published in Circulation. Peripheral blood was drawn from patients who have a genetic variant that confers protection against cardiotoxicity. Stem cells were isolated and grown into cardiomyocytes which could then be exposed in vitro to the cardiotoxic drug (doxorubicin) to validate the strong association between gene and adverse drug reaction demonstrated in earlier published research. This work has taken the genetic findings from a robust statistical association through to drug discovery. With this human cell-tested data, a clinical trial of an approved drug, repurposed to protect against doxorubicin cardiotoxicity, is now being planned.
Such models can show that a strong association between a gene and drug outcome are more than a mere observed association; they provide biological evidence that helps to identify the mechanistic basis of the adverse reaction and ultimately point to potential solutions for safer products and practices (such as who might be best to avoid that particular drug).
Our plan is to do the same thing with vaccines and adverse events following immunisation by using case-control methods to identify genomic factors that are highly associated with the adverse outcome. We will then use this evidence to identify the biology that underlies the mechanism of a specific vaccine-induced harm. Such information can then be used to better understand the reaction, how to prevent it and how we might treat it. Finding the cause is also the first step for creating safer vaccines. How do you solve a safety problem if you don’t know the cause?
Key points- Ordinarily, vaccines are very safe. Rarely, vaccines can cause serious adverse events. - For some types of adverse drug and vaccine events genetic variants that place some individuals at risk - Modern techniques have made it possible to study the genetics underpinning these events. These - This is a great step towards the development of even safer vaccines. |
About the author
Dr. Carleton is the:
- Lead for the GVDN Global COVID Vaccine Safety (GCoVS) project genomics/adversomics protocol work group
- Professor and Chair, Division of Translational Therapeutics, Department of Pediatrics, Faculty of Medicine, The University of British Columbia, Canada
- Senior Clinician Scientist, BC Children’s Hospital Research Institute, Canada
- Director, Pharmaceutical Outcomes Programme, BC Children’s Hospital, Canada
- Director, Therapeutic Evaluation Unit, Provincial Health Services Authority, Canada